The Fine Print – Registrations, Exemptions and Licensing
go.ncsu.edu/readext?1004950
en Español / em Português
El inglés es el idioma de control de esta página. En la medida en que haya algún conflicto entre la traducción al inglés y la traducción, el inglés prevalece.
Al hacer clic en el enlace de traducción se activa un servicio de traducción gratuito para convertir la página al español. Al igual que con cualquier traducción por Internet, la conversión no es sensible al contexto y puede que no traduzca el texto en su significado original. NC State Extension no garantiza la exactitud del texto traducido. Por favor, tenga en cuenta que algunas aplicaciones y/o servicios pueden no funcionar como se espera cuando se traducen.
Português
Inglês é o idioma de controle desta página. Na medida que haja algum conflito entre o texto original em Inglês e a tradução, o Inglês prevalece.
Ao clicar no link de tradução, um serviço gratuito de tradução será ativado para converter a página para o Português. Como em qualquer tradução pela internet, a conversão não é sensivel ao contexto e pode não ocorrer a tradução para o significado orginal. O serviço de Extensão da Carolina do Norte (NC State Extension) não garante a exatidão do texto traduzido. Por favor, observe que algumas funções ou serviços podem não funcionar como esperado após a tradução.
English
English is the controlling language of this page. To the extent there is any conflict between the English text and the translation, English controls.
Clicking on the translation link activates a free translation service to convert the page to Spanish. As with any Internet translation, the conversion is not context-sensitive and may not translate the text to its original meaning. NC State Extension does not guarantee the accuracy of the translated text. Please note that some applications and/or services may not function as expected when translated.
Collapse ▲Webinar Highlights
On-Farm Requirements
Where does your farm fall under the FSMA Produce Safety Rule?
Farms can fall into one of three categories: covered farms, farms eligible for qualified exemption & modified requirements or the farm is not covered under the Produce Safety Rule.
- Covered Farms – more than $638,491 in annual food sales for last 3 years AND most sales are not to end consumer.
Covered small farms – annual food sales for last 3 years falls between$250,000-$638,491
Covered very small farms – annual food sales for last 3 years falls between ($31,925-$250,000)
- Farms eligible for qualified exemption & modified requirements have average food sales under $638,491 for the last three years AND the majority of food sales must be made to Qualified End Users (QEU) during that time frame.
- Farms not covered by the Produce Safety Rule average less than $31,925 in produce sales for last 3 years AND the majority of food sales must be made to Qualified End Users (QEU) during that time frame.
Qualified End Users are the consumer of the food (regardless of location) and include your farm, farmers market, CSA and/or via internet sales. Restaurants and grocery stores (that you deliver to directly) within 275 miles of your farm
NC State Extension has created a worksheet that will help guide you through determining your farm’s status.
 Exemptions to the Produce Safety Rule
Exemptions to the Produce Safety Rule
- Farms that only grow rarely consumed raw produce. These are fruits and vegetables that are almost always cooked before being consumed.
- Farms that only grow produce that will be commercially processed and in which there will be a kill step.
- If exempt from the Produce Safety Rule, you are still fully liable for growing safe produce under the Federal Food, Drug & Cosmetic Act.
FDA Food Facility Requirements
What is an FDA Food Facility?
A food facility is any establishment, in one physical location or mobile, that manufactures, processes, packs and/or holds foods for human consumption. Also applies to multiple company entities processing at a shared facility.
Why do I need to register my food facility?
The Public Health Security and Bioterrorism Preparedness and Response Act of 2002 (the Bioterrorism Act) directs the Food and Drug Administration (FDA), to take steps to protect the public from a threatened or actual terrorist attack on the U.S. food supply and other food-related emergencies. These emergencies could include your product, or ingredients of your product, being identified in an outbreak or recall event.
Food Facility Registration Basics
- There is no cost to register, renew or update food facility registrations.
- Each facility location must gain a unique facility identifier (UFI). Dun & Bradstreet is currently the only acceptable source of a UFI, this service is also free of charge.
- Companies will also need to include their facility name, address or location, activity types and food product categories (if they are manufacturing or processing food).
FDA Food Facility Activity Types
| Manufacturer or Processor | Farm mixed-type facility | Acidified foods processor | Low-acid canned foods processor |
| Packer or Repacker | Labeler or Relabeler | Salvage operation or reconditioning | Ambient, refrigerated or frozen warehouse or holding facility |
| Interstate conveyance caterer | Contract sterilizer | Animal food warehouse or holding facility | Other, must specify |
Food Product Categories
| Baby (Infant and Junior) Food Products | Cheese and Cheese Product Categories: Soft, Ripened Cheese; Semi-Soft Cheese; Hard Cheese; Other Cheeses and Cheese Products; | Dietary Supplement Categories: Proteins, Amino Acids, Fats and Lipid Substances; Animal By-Products and Extracts; Herbals and Botanicals | Fishery/Seafood Product Categories: Fin Fish, Whole or Filet; Other Shellfish; Ready to Eat (RTE) Fishery Products; Processed and Other Fishery Products; Molluscan Shellfish |
|
Fruit and Fruit Products: Fresh Cut Produce; Raw Agricultural Commodities; Other Fruit and Fruit Products |
Fruit or Vegetable Juice, Pulp or Concentrate Products | Nuts and Edible Seed Product Categories: Nut and Nut Products; Edible Seed and Edible Seed Products | Shell Egg and Egg Product Categories: Chicken Egg and Egg Products; Other Egg and Egg Products |
|
Vegetable and Vegetable Product Categories: Fresh Cut Products; Raw Agricultural Commodities; Other Vegetable and Vegetable Products |
Grain or Grain Products (i.e., barley, grain sorghums, maize, oat, rice, rye, wheat, other grains or grain products) | Oilseed or Oilseed Products (i.e., cottonseed, soybeans, other oilseeds or oilseed products) | Alfalfa Products or Lespedeza Products |
| Milk Products | Citrus Products | Miscellaneous or Special Purpose Products | Processed Animal Waste Products |
| Animal By-Products and Extracts | Distillery Products | Molasses or Molasses Products | Technical Additives |
| Amino Acids or Related Products | Fats or Oils | Marine Products | Vitamins or Vitamin Products |
| Animal Protein Products | Enzymes | Peanut Products | Yeast Products |
| Botanicals and Herbs | Herbals and Botanicals | Fermentation Products | Minerals or Mineral Products |
| Chemical Preservatives | Forage Products | Brewer Products | Human food by-products not otherwise listed* |
Qualified Facility Attestation
The FDA provides qualifications for smaller businesses that lift some requirements of 21 CFR 117. Qualified facilities are only subject to subparts A, B & F.
Subpart A – General Provisions
Subpart B – Current Good Manufacturing Practices for Human Foods
Subpart F – Records
Companies must file their attestation with the FDA and keep records to prove revenue. Determining if you’re qualified facility can be calculated using tax forms (IRS Form 1120), accounting documents, and/or invoices and bills of lading.
Important Registration Dates & Timelines
Registration Activity |
Timeline |
| Initial FDA Food Facility Registration | Prior to starting operations |
| FDA Food Facility Registration Renewal | Biannually, on even-numbered years. Between October 1 & December 31st |
| Updates to required registration information | Within 60 calendar days |
| Change of ownership | Former owner must cancel within 60 calendar days and new ownership must register prior to starting operations |
| Qualified Facility Attestation |
Assess facility status annually by July 1 AND Submit form FDA 3942a when renewing FDA Facility Registration biannually |
| If no longer a Qualified Facility, must submit an FDA Form 3942a by the end of July |
Exemptions from Registering as a Food Facility
| Farms | Beekeeping and honey production | Retail food establishments | Home processors |
| Grocery stores & Supermarkets | Convenience stores | Vending machines | Transportation vehicles |
| Meal-kit type services | Non-profit establishments | Farmers Markets* | Fishing vessels ** |
* When the majority of product sold is to the consumer (not wholesale) ** When the vessel is not processing seafood onboard
NC Licenses for FDA-regulated Food Facilities
NCDA&CS’s Food Program required licensing for the following industries*:
–Grade A milk operation (permit)
–Milk haulers & testers
–Cheese and butter processors
–Frozen dessert manufacturers
*List excludes any type of business or commerce-based licenses like a NC business license or Tax ID
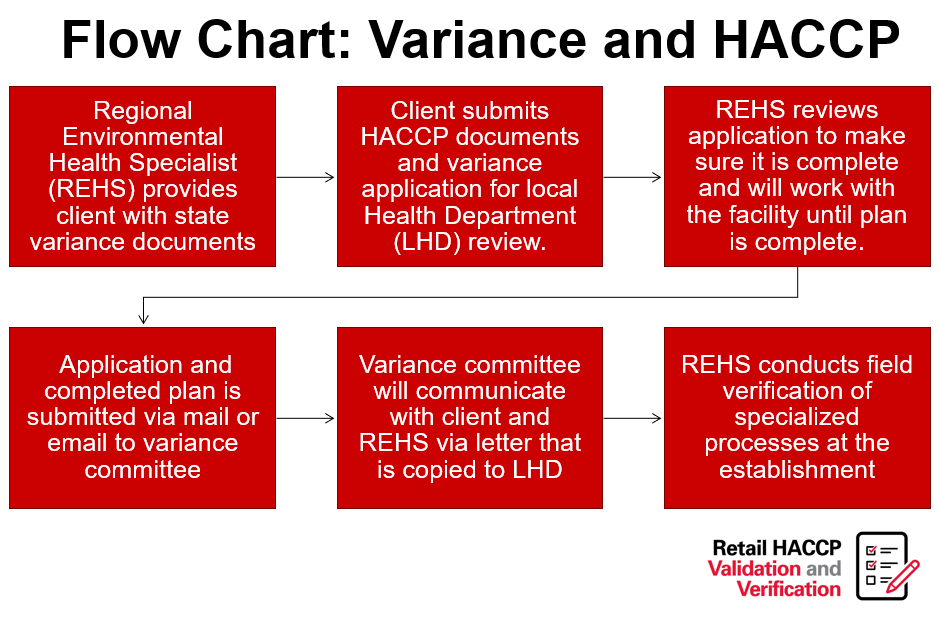
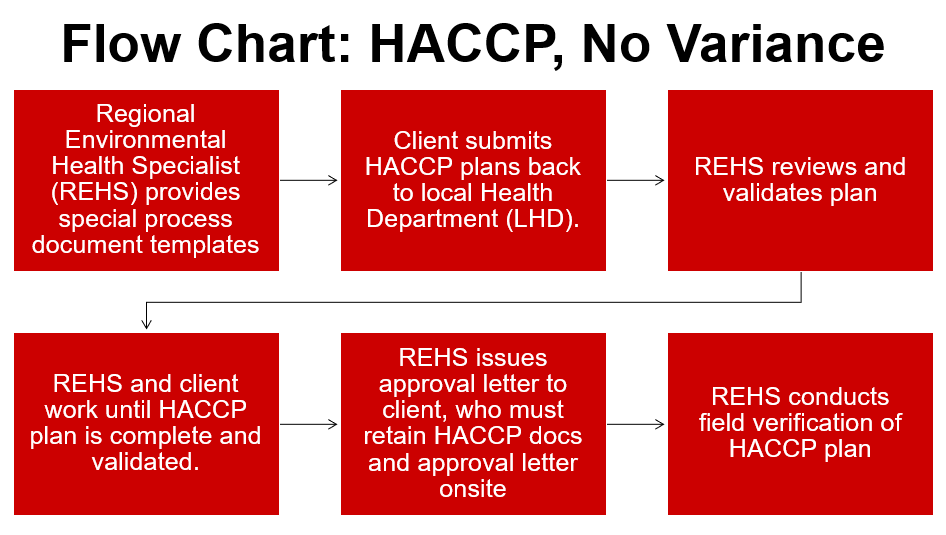
Retail Food Variance Requirements
Retail food establishments, including restaurants, buffets, caterers and other food service establishments, at times, want to produce foods that are either high-risk or outside of the typical parameters for food safety, as outlined in the NC Food Code. The North Carolina Department of Health and Human Services has identified many of these high-risk items and has created a system that allows their production. This system includes a written application, review and implementation processes required for companies engaging in high-risk processing (note flow charts further in this post).
“Variance” means a written document issued by the regulatory authority that authorizes a modification or waiver of one or more requirements of this Code if, in the opinion of the regulatory authority, a health hazard or nuisance will not result from the modification or waiver.”
Variances in North Carolina require a Hazard Analysis and Critical Control Points (HACCP) plan. HACCP plans are a systematic, hazard-based system that ensures risks are controlled in the processing of food items. These plans shows the processing of foods is equal in safety to what is already allowed in the NC Food Code Manual. Lastly, HACCP plans are specific to the product, processing and facility.
Identified Special Processes
| Special Process | Variance Required | Variance Exceptions | HACCP Plan Required | HACCP Plan Exceptions |
| Reduced Oxygen Packaging | YES/NO | Methods under 3-502.12 with validated process | YES | Held at 41°F or less and removed from package within 48 hours |
| Sprouting | YES | YES | ||
| Custom Processing for Personal Use | YES | YES | ||
| Operating Live Molluscan Shellfish Tank | YES | YES | ||
| Curing, Drying, Smoking of Fish | YES | Smoking for flavor enhancement, color or part of cooking process | YES | Smoking for flavor enhancement , color or part of cooking process |
| Curing, Smoking of meat/poultry | YES | Smoking for flavor enhancement , color or part of cooking process | YES | Smoking for flavor enhancement , color or part of cooking process |
| Drying of Meat/Poultry | YES | YES | ||
| Fermentation of Sausages | YES | YES | ||
| Adding Components to Extend Shelf life | YES | YES | ||
| Juice processing and packaging | NO | A performance standard of 5 log reduction required | YES | Warning label can be applied in lieu of HACCP plan |
View NC DHHS’s full list of identified products requiring additional food safety considerations.
View NC DHHS’S variance decision tree flow chart.
More examples of when a variance is required:
1. Smoking food for food preservation rather than a method of flavor enhancement;
2. Curing food;
3. Using food additives or adding components such as vinegar to address pH:
- As a method of food preservation rather than as a method of flavor enhancement; or
- To render a food so that it is not potentially hazardous (Time/Temperature Control of Safety Food);
4. Packaging food using a ROP method except as specified under Section 3-502.12;
5. Operating a molluscan shellfish life-support system display tank used to store or display shellfish offered for human consumption;
6. Custom processing animals for personal use (not for sale/service in a Food Establishment);
7. Preparing food by another method that is determined to require a variance; or
8. Sprouting seeds or beans.
Examples of when a HACCP plan, but not a variance is required:
1. A TCS food that is packaged using reduced-oxygen packaging (ROP) method, maintained at 41°F or less and meets at least one of the following criteria:
- Has a water activity (Aw) of 0.91 or less;
- Has a pH of 4.6 or less;
- Is a meat or poultry product cured at a food processing plant regulated by the NCDA&CS Meat & Poultry Inspection Division or USDA; or
- Is a food with a high level of competing organisms, such as raw meat, poultry or vegetables.
2. Fish that is frozen before, during, and after packaging using a ROP method (3-502.12)
3. Food that is prepared and packaged using a cook-chill or sous vide method (3-502.12);
4. Specific cheeses that are packaged using a ROP method (3-502.12);
5. Juice packaged in a food establishment without a warning label (3-404.11);
6. Unpackaged juice prepared in a food establishment serving a highly susceptible population (3-801.11)
Contact our Speakers:
| Elena Rogers | Horticulture Area Specialized Agent | elena_rogers@ncsu.edu | https://ncfreshproducesafety.ces.ncsu.edu/ |
| Mary Yavelak | Food Safety Extension Associate | mkyavela@ncsu.edu | https://foodsafety.ces.ncsu.edu/ |
| Kate Nicholas | Food Safety Extension Associate | katenicholas@ncsu.edu |
https://foodsafetyprocessors.ces.ncsu.edu/ and |
Webinar hosted on May 17, 2024
![]() “This work was supported by the intramural research program of the U.S. Department of Agriculture, National Institute of Food and Agriculture, Food Safety Outreach Program 1030908. The findings and conclusions in this preliminary publication have not been formally disseminated by the US Department of Agriculture and should not be construed to represent any agency determination or policy.”
“This work was supported by the intramural research program of the U.S. Department of Agriculture, National Institute of Food and Agriculture, Food Safety Outreach Program 1030908. The findings and conclusions in this preliminary publication have not been formally disseminated by the US Department of Agriculture and should not be construed to represent any agency determination or policy.”